Differentiation
Wearable and electrode applications: Flexcon® Omni-Wave™ eases converting, saves money, and extends shelf life of biosensing devices
The growth of wearable sensor technology is evident in both the multitude of measured characteristics and the enhanced sensitivity of wearable medical devices. Until now, hydrogels were the only means of signal transmission, but that changed with the introduction of Flexcon® Omni-Wave™, a revolutionary new material that eliminates the need for hydrogel and the headaches and costs associated with converting it, all while improving patient comfort. Flexcon recently worked with Innovize to quantify the cost benefits and ease of converting with Omni-Wave™ compared to hydrogels. The results are in!
- Longer shelf life
- Faster and easier converting
- No special handling requirements
- Lower cost packaging requirements
- Reduced shipping costs
- Longer out-of-pack life of finished devices
- No hydrogel-related skin reactions for patients
What is Omni-Wave™?
Omni-Wave™ is disruptive dry biosensing technology that utilizes a skin-friendly, medical-grade conductive adhesive that requires no hydrogel or Ag/AgCl to get a signal, offering a hydrogel-free patient experience. Independent clinical studies show that the performance of Omni-Wave™ is equivalent to hydrogel-based device components. Humidity does not impact function, and out-of-pack life is two years compared to three to six months for hydrogel. Omni-Wave™ is an approved component in customer 510(k) and CE-marked finished good medical devices and meets internationally accepted standards for quality.
“Flexcon is proud to be bringing this disruptive technology to market that will benefit both device manufacturers in terms of costs and manufacturability, as well as the comfort of patients.”
-Amit Roy, Business Development Manager Healthcare, Flexcon
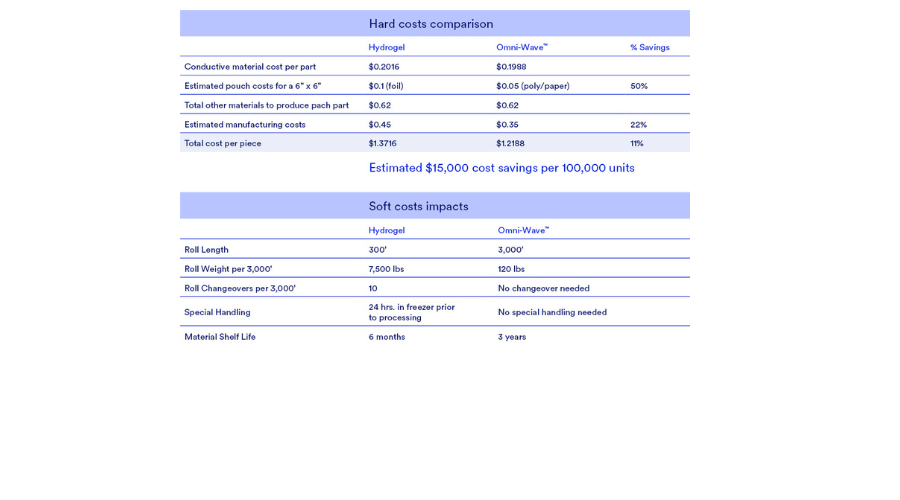
Converting comparison
Of the five materials used in a typical device (non-woven material, carbon-vinyl film, extended-release liner, conductive material, and pouch), two things have changed: replacement of hydrogel with Omni-Wave™ and use of a less expensive paper/poly pouch.
Following is a general cost comparison of Omni-Wave™ vs. a traditional hydrogel based device on the production of 100,000 units via rotary converting on a Delta ModTech Converting Press.
Converting efficiencies and savings
- Omni-Wave™ uses less expensive packaging while offering an out-of-pack life of two years.
- Total manufacturing costs are lower.
- Longer 3,000-foot rolls mean fewer roll changes.
- Omni-Wave™ is easier to process.
- Omni-Wave™ requires no special handling.
- Omni-Wave™ is lighter weight, reducing logistical issues and shipping costs at scale.
- Omni-Wave™ offers four to eight times longer shelf-life, reducing the risk of costly waste.
- Less expensive EtO sterilization may be used with Omni-Wave™.
“Reduced costs and waste, and no production headaches are game changers. Achieving a better patient experience is an added bonus.”
-Stephanie Wright, Marketing Manager, Innovize
The bottom line
While hydrogels have been the standard in healthcare for decades, they are cumbersome and costly to run due to numerous changeovers, greater potential for waste, expensive packaging requirements, and special handling requirements when compared to Omni-Wave™. With comparable raw material costs, this process will be the low-cost option for any mid- to high-volume wearable device, especially if the packaging can be fully automated, as is done at Innovize.
Hydrogels still have their place in wearable sensors; however, the Omni-Wave™ material offers both savings in total cost and longer shelf life if it meets the requirements for your specific application, thereby resulting in higher net margins per unit.
Innovize and Flexcon would be thrilled to collaborate on your next wearable sensor project.
We can’t wait to connect!