Success Stories
Ampoule Tracking Issue Solved
Diagnostic Provider Collaborates with Flexcon on Ampoule Labeling Challenge
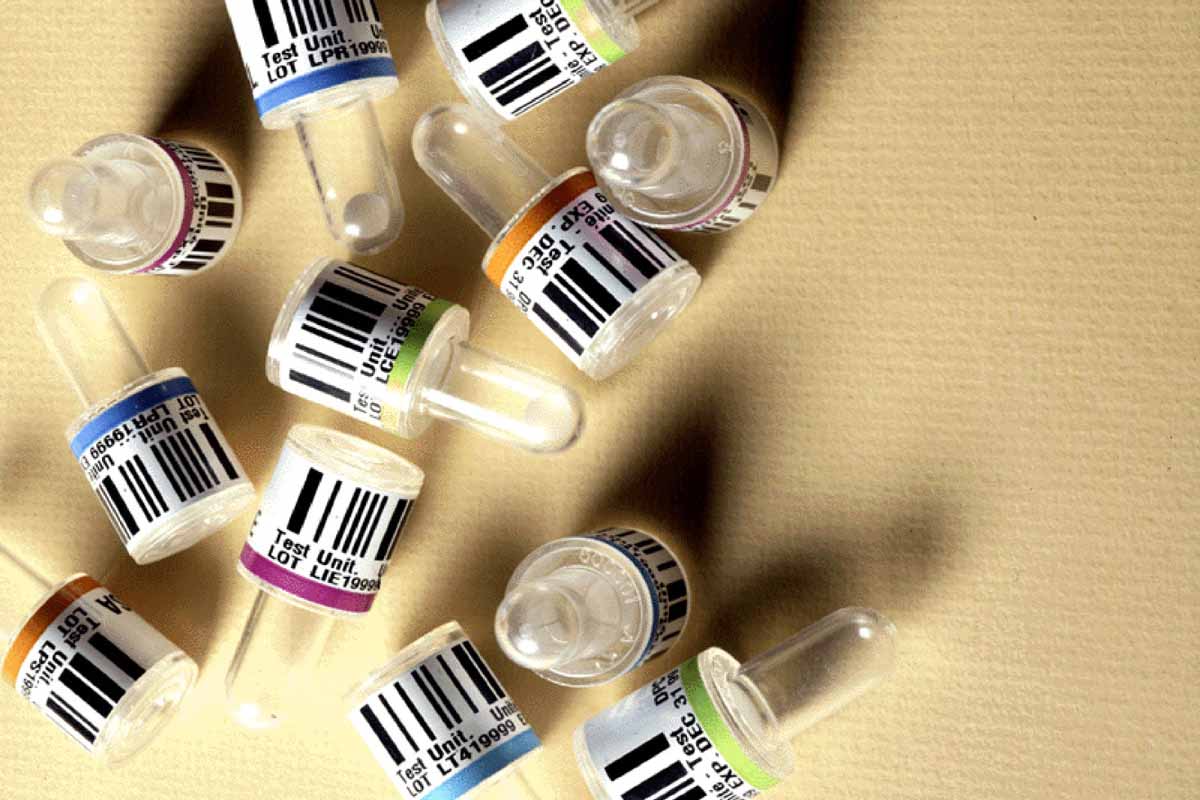
The Project
Specimen testing in special ampoules has been an important tool in diagnosing cancer, the travel of the disease, and effectiveness of treatment. The labels on diagnostic ampoules carry pertinent information about the patient as well as quantifying research variables. When a day’s worth of testing can accumulate more than 150,000 specimens, meticulous labeling is vital to track the results.
Recently, a colon cancer diagnostic provider encountered difficulties with its ampoule labels. The paper label they had been using would gradually peel off when exposed to centrifuge forces and cryogenic temperatures, losing all traceability of a specimen to the patient and losing relevant research data. When one ampoule in a series of testing is untraceable, the entire series must be rejected and new samples must be taken from the patient. Given the lab’s requirements for organization and efficiency, the loss of labels in the experimental process is not only inconvenient to lab workers and patients, it is damaging to the laboratory’s reputation. In colon cancer screenings, a lost label can have even more serious consequences; a wrong specimen could lead to misdiagnosis.
The Challenge
The ampoules are exposed to rigorous conditions and it is a challenge for labels to adhere permanently. Colon cancer is screened by taking a tissue sample from a patient and injecting it into an ampoule that already contains an agent. When centrifuged, the sample mixes with the agent and the result shows whether there is cancer. The ampoule is manufactured from a styronated resin which contains agents that migrate to the surface. This surface contaminate creates a very low surface energy, therefore increasing the challenge of finding the proper adhesive that will allow for a permanent bond of the barcode tracking label.
Additional challenges other then the adhesive include abrasions to the outside of the ampoule that result from shipping, the automated application process in a clean room environment, and the need for high-definition barcode capability to meet the scannability and compliance requirements set by the Food & Drug Administration. In order to be readable, these barcodes need an extremely high-definition, dense code; however this application also involves small print on a tight diameter.
The Process
Flexcon, a global manufacturer of pressure-sensitive film products, proposed an opaque polypropylene film. The film had to be conformable enough to go around the ampoule, but rigid enough to automatically dispense. The opacity was critical in producing the appropriate black and white contrast rating necessary for optimal readability and “A” grade ANSI barcode scans. The adhesive was the next challenge. Flexcon chose their V-367 adhesive to combat the low surface energy of the ampoules. V-367 is an aggressive permanent adhesive with excellent resistance to chemicals, heat, and humidity.
The Material
For maximum printability, Flexcon carries a variety of high performance topcoatings for thermal transfer printing. In the case of the colon cancer diagnostic provider, Flexcon’s TC-387 was the topcoat choice for the application. Flexcon’s TC-387 is compatible with a wide range of resin and wax/resin ribbons and offers superior abrasion resistance. More than just enhancing the ability to print, the topcoat protects the label. It is durable while resistant to blood and other possible exposures. The topcoat had to succeed by being soft enough to print, but durable enough to perform under varying conditions.
The Result
The construction was backed with a polyester release liner, which aided in the dispensing of the label onto the ampoule. The liner was crucial in minimizing the amount of human contact with the automatic label application equipment and ampoules inside the clean room environment.
The cancer diagnostic provider tested the Flexcon construction on 2,000,000 vials. Out of these, only two labels began to peel.
Flexcon was successful in providing a labeling film, topcoat, and medical adhesive that stand up to the inner and outer elements affecting the identification labels of the ampoule. The colon cancer diagnostic provider continues to use this labeling product as a way to assure the precision and accuracy that is the lifeblood of their business.